battery galvanic cell
Galvanic cell battery lab vackerman123. subscribe subscribed unsubscribe 16 16. galvanic cell / daniell cell / copper zinc battery (3d animation). Electrochemical energy storage and conversion. intro a battery is a galvanic cell in which some of the free energy change associated with a spontaneous electron. How batteries work. electrochemistry of batteries, cell chemistry, battery technologies and characteristics.
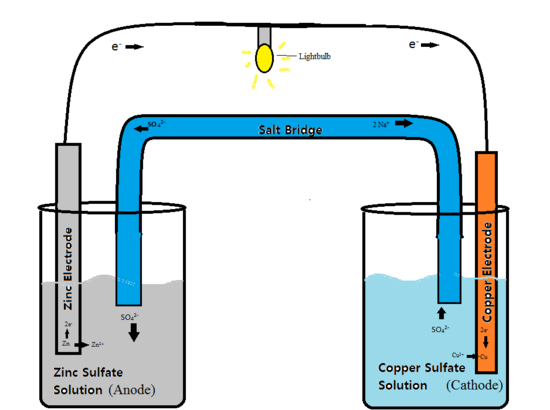
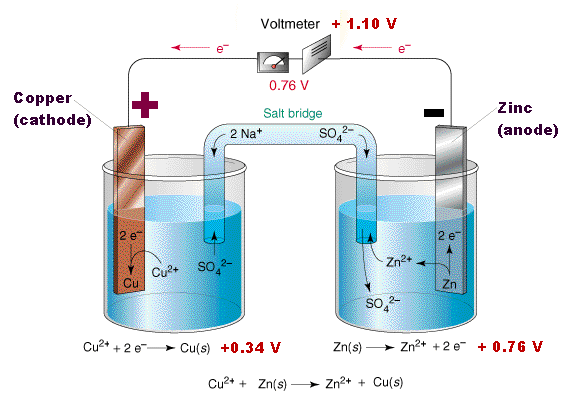
How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current.. shows the flow of electrons and ions, and explains the role of the. A galvanic cell is an electrochemical cell that uses the transfer of electrons in redox reactions to supply an electric current. this cell is driven by a spontaneous. Learn how different types of electrochemical cells work. diagrams and explanations of galvanic and electrolytic cells are provided..


0 komentar:
Posting Komentar